Where there is steel, there is potential for corrosion. Cars, trucks, bridges, and skyscrapers get their strength from steel. The same goes for steel cable, pipe, railcars, and guardrails. Replacing and repairing them is expensive. The annual direct cost of corrosion is approximately 3.1% of a country’s gross domestic product (GDP, Shaw & Kelly, 2006). The National Association of Corrosion Engineers estimates that the global cost of corrosion is $2.5 trillion (NACE International, 2016). That is more than the GDP of Italy, Canada, South Korea, and Russia (Global PEO Services, n.d.). Corrosion affects the bottom line and can pose a real danger to society.
Not so Hidden Costs of Corrosion
Repair and replacement costs pale compared to the environmental and human tragedies that have occurred in history.
- In 1984, in Bhopal, India, a steel pipe corroded and allowed water to leak into a phosgene tank. The rusted iron and water served as a catalyst for the reaction and blew the plant apart, resulting in the death of 23,000 people (Hansson, 2011).
- In 2006, a corroded North Alaskan pipeline sprung a leak and covered almost two acres of land with crude oil (Hansson, 2011).
- In 2018, corroded steel cables supporting a bridge in Genoa, Italy, failed, resulting in the death of 43 people (Cowlishaw, 2021).
Corrosion 101
The most common type of corrosion, or rust, is the reddish color usually seen on steel as it oxidizes. It typically starts as tiny spots and progresses to a light rash. The rust particle grows and flakes off the base metal during the process. Eventually, oxygen and water will cause the iron in the steel to convert to rust, ultimately disintegrating it. Another type of rusting, galvanic corrosion, is a little more complicated but can be explained by describing how a battery produces electrical energy.
Battery Power 101
Virtually every portable electronic device we own uses batteries for its power. Electric cars, digital watches, cell phones, and remote-control devices are a marvel of modern technology but could not function without using battery energy. A battery stores chemical energy and releases it as electrical energy. A chemical reaction occurs as electrons are transferred from one material to another through an external circuit (Bhatt et al., n.d.). This movement of electrons in the battery provides the electric current that powers our electronic devices.
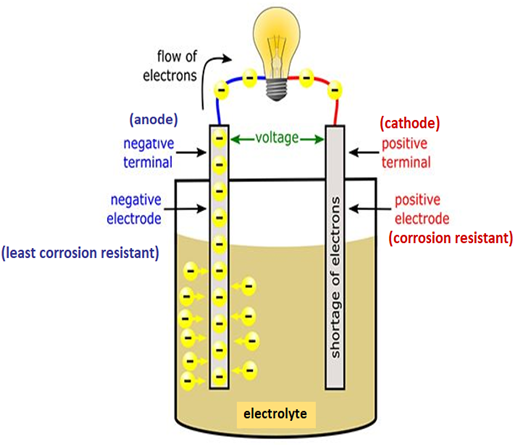
A more technical description is needed to understand a battery’s relationship to corrosion and rust fully. Figure 1 shows an ordinary battery consisting of two metals, the cathode (positive) and the anode (negative), and an electrolyte between them. One of the metals prefers to give up electrons, and the other likes to receive them. The plus terminals are corrosion-resistant metals, while the minus terminals are the least corrosion-resistant metals.
Electrons build up at the anode of the battery due to chemical reactions. The electrolyte transfers electrical charge between the two metals by acting as a chemical medium (Bates, 2012), allowing electrons to flow from the anode to the cathode (Rosendo, 2011). When the battery is put in a device, a connection is made, and the electrons can reach the cathode and supply energy. Eventually, the depleted electrolyte stops the movement of ions through it, electrons stop flowing, and the battery dies (Woodford, 2021).
Figure 1
Battery Diagram

Note. Adapted from “Electronics for kids for dummies,” by C. Shamieh, 2016.
Galvanic Series and Dissimilar Metals
The noblest or more corrosion-resistant metals are cathodic, and the least noble or corrosion-resistant metals are anodic (D’Antonio, 2017). A metal’s nobility is a critical characteristic that distinguishes it from other metals. The more noble a metal is, the more it resists corrosion. A metal’s place on the galvanic scale represents how easily it gives up electrons. The further apart two metals are on the galvanic scale, the more dissimilar they are. As shown in Figure 2, a galvanic series chart illustrates metals in order of their corrosion resistance.
Figure 2
Galvanic Series
Note. From “Galvanic corrosion,” by C. Frangos, 2021, (https://dctech.com.au/2021/07/13/galvanic-corrosion/).
Galvanic Corrosion
The most basic form of galvanic corrosion occurs when dissimilar metals come into contact with each other and an electrolyte, for example, water (D’Antonio, 2018). Like the battery example above, electrons flow between the two dissimilar metals to produce a small current. Figure 3 shows the diagram of a simplified galvanic cell. Galvanic corrosion cannot occur if one of the four components is missing (Construction Specifier, 2020).
Figure 3
Galvanic Cell
Note. From “Avoiding galvanic corrosion with dissimilar metals,” by Construction Specifier, 2020, (https://www.constructionspecifier.com/avoiding-galvanic-corrosion-with-dissimilar-metals/2/).
A metal’s corrosion rate depends on its distance from the next metal in the ranking in the galvanic series. The greater the distance between the metals, the greater the corrosion rate (Weber, 2003). For example, aluminum brackets stored outside in the weather will corrode slowly. Connecting one of those aluminum brackets to a piece of iron would cause it to corrode more quickly because it is the most active metal (or anode). A similar result would happen with an aluminum propeller bolted to a steel ship. The aluminum propeller would rust much faster than the steel portion of the vessel.
There is also a relationship between the relative amount of each metal and corrosion rate. A large amount of less active metal in contact with a small amount of more active metal causes the more active metal to corrode quicker (Weber, 2003). A linear relationship exists between the size differences of the cathode and the anode. The rate at which the anode corrodes increases as the size difference increases (Rosendo, 2011). This principle only applies if the less active metal is larger than the more active metal. If the anode is larger than the cathode, there is an inverse relationship, and the corrosion rate decreases (Rosendo, 2011).
Figure 4 shows a real-world example of corrosion caused by dissimilar metals. The active steel roofing screws have a relatively small area compared to the larger size of the less active zinc-coated galvanized roofing. As can be seen, only the screws are rusting, while the galvanized (zinc-coated) decking is largely unaffected.
Figure 4
Galvanized Roofing

Note. From “Dissimilar metal roof sheeting – galvanic corrosion,” by Ambrose Building, 2017, (https://ambrosebuilding.com.au/insurance-repairs/dissimilar-metal-roof-sheeting-galvanic-corrosion/).
Conclusion
There are ways to prevent or slow down galvanic corrosion. When connecting metal to metal, try to use the same type of material. Remember to use the galvanic series chart when using the same metal is impossible. Avoid using an active metal like aluminum to connect an inactive metal like steel. Aluminum fasteners, for example, have a relatively smaller area than steel and are dissimilar to the extent that accelerated corrosion will occur (Weber, 2003). Insulate between the two metals if possible. Whenever feasible, design for the easy removal and replacement of the anodic piece or part (Hansson, 2011). Painting with an anticorrosive coating can prevent corrosion if applied as a barrier between the two metals.
Corrosion is a ubiquitous problem that results in property loss and sometimes human life. In one form or another, it has plagued humanity for millennia. It would be almost impossible to go outdoors and not find something showing signs of it. We are developing new ways to stop or slow corrosion all the time. One day it may be a thing of the past, but until then, remember the galvanic series and apply your knowledge to help make it a distant memory.
References
Ambrose Building. (2017). Dissimilar metal roof sheeting — galvanic corrosion. https://ambrosebuilding.com.au/insurance-repairs/dissimilar-metal-roof-sheeting-galvanic-corrosion/
Bates, M. (2012). How does a battery work? MIT School of Engineering. https://engineering.mit.edu/engage/ask-an-engineer/how-does-a-battery-work
Bhatt, A., Forsyth, M., ShihWithers, R., & Wang, G. (n.d.). How a battery works. Australian Academy of Science. https://www.science.org.au/curious/technology-future/batteries
Construction Specifier. (2020). Avoiding galvanic corrosion with dissimilar metals. https://www.constructionspecifier.com/avoiding-galvanic-corrosion-with-dissimilar-metals/2/
Cowlishaw, S. (2021). 5 disasters caused by corrosion. LinkedIn. https://www.linkedin.com/pulse/5-disasters-caused-corrosion-samuel-cowlishaw/
D’Antonio, S. (2017). Noble efforts. Cruising World, 43(3), 59.
D’Antonio, S. (2018). Metal matters. Cruising World, 68–71.
Frangos, C. (2021). Galvanic corrosion. Dynamic Composite Technologies. https://dctech.com.au/2021/07/13/galvanic-corrosion/
Global PEO Services. (n.d.). Top 15 countries by GDP in 2022. https://globalpeoservices.com/top-15-countries-by-gdp-in-2022/
Hansson, C. M. (2011). The impact of corrosion on society. Metallurgical and Materials Transactions A, 42(10), 2952–2962. https://doi.org/10.1007/s11661-011-0703-2
NACE International. (2016). International measures of prevention, application, and economics of corrosion technologies study. https://doi.org/http://impact.nace.org/documents/Nace-International-Report.pdf
Rosendo, A. (2011). Corrosion comes in different forms. Power Engineering. https://www.power-eng.com/emissions/air-pollution-control-equipment-services/corrosion-comes-in-different-forms/
Shamieh, C. (2016). Electronics for kids for dummies (1st ed.). John Wiley & Sons.
Shaw, B. A., & Kelly, R. G. (2006). What is corrosion? The Electrochemical Society Interface, 15, 24–26. https://doi.org/https://www.electrochem.org/dl/interface/spr/spr06/spr06_p24-26.pdf
Weber, R. (2003). How to deal with dissimilar metals. Refrigerated Transporter, 39(27), 33. https://www.trailer-bodybuilders.com/fabrication/article/21741366/how-to-deal-with-dissimilar-metals
Woodford, C. (2021). Batteries. Explainthatstuff!. https://www.explainthatstuff.com/batteries.html